Abstract
Introduction Therapy-related acute myeloid leukemia (t-AML) and AML with myelodysplasia-related changes (AML-MRC) are associated with poor outcomes due to older age at diagnosis and adverse-risk cytogenetics and molecular features and, therefore, treatment of these groups of patients remain a challenge. There is a paucity of real-world data on outcomes of t-AML and AML-MRC treated with 7+3, hypomethylating agents (HMA) with or without venetoclax (VEN) or CPX-351 (Vyxeos).
Methods We conducted a single-center retrospective cohort study to compare overall survival (OS), event-free survival (EFS), and composite complete response (CCR) of patients with t-AML or AML-MRC treated with first-line 7+3, HMA, HMA+VEN or Vyxeos. Therapy-related myelodysplastic syndrome were not included. OS was defined from day 1 of initial chemotherapy until death from any cause. Patients were not censored at the time of allogeneic transplant. Sixty and 90-day treatment-related mortality were estimated as surrogates for treatment toxicity. Propensity score modeling was used to adjust for baseline characteristics (age, AML subtype, comorbidities, performance status, cardiac ejection fraction, cytogenetics, and mutations).
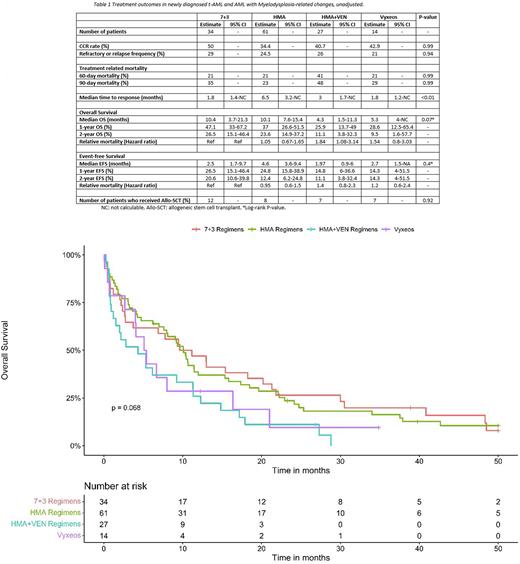
Results Of 488 consecutive AML patients treated at the University of Maryland between 2013-2021, 136 patients had t-AML or AML-MRC and received one of the above therapies. 40% were female. The median age of the cohort was 67.5 [IQR: 60.9-73.2]. The median age of patients who received 7+3 (n=34) was 63.4 years, HMA (n=61) 70.3 years, HMA+VEN (n=27) 72.6 years, and Vyxeos (n=14) 62.2 years (age P-value= 0.1). 42 and 17 patients received decitabine in the HMA and HMA+VEN arms, respectively. None of the patients treated with 7+3 or Vyxeos received HMA prior to AML diagnosis. However, 3 and 6 patients received HMA in the HMA and HMA+VEN arms prior to AML diagnosis, respectively. Both 7+3 and HMA were given mostly before 2019 (7+3: 88%; HMA: 81%) compared to HMA+VEN and Vyxeos, which were given mostly in years 2019 and after (HMA+VEN: 74%; Vyxeos: 71%) (P<0.01). The median follow-up was 73.5 months (mo). Table 1 demonstrates unadjusted outcomes. Figure 1 demonstrates the unadjusted OS of treated groups. Using propensity score for adjustment, there was no difference in median OS or CCR in patients treated with 7+3 vs. HMA (11.2 vs. 10.6 mo, P=0.63; 42% vs. 31%, P=0.38). However, patients treated with HMA+VEN had worse adjusted m-OS than patients treated with 7+3 (5.3 vs. 20.2 mo, P=0.02) or HMA alone (5.3 vs. 10.1 mo, P=0.02). Patients treated with Vyxeos had a statistically similar adjusted-median OS to those treated with 7+3 (5.1 vs. 15.4 mo, P=0.34) and a trend toward a worse outcome than those treated with HMA alone (5.3 vs. 10.1 mo, P=0.07).
Conclusion The outcomes of t-AML and AML-MRC remains poor. In t-AML and AML-MRC, using 7+3 and HMA resulted in similar OS; however, improved outcomes compared to HMA+VEN. Almost 50% of patients treated with HMA+VEN died in the first 90 days, possibly related to treatment toxicity. Further studies are needed to determine the role of VEN combination with HMA in t-AML and AML-MRC. Moreover, further real-world, multi-institutional data are needed to evaluate the effectiveness of Vyxeos in t-AML and AML-MRC.
Disclosures
Baer:Ascentage: Research Funding; Kura Oncology: Research Funding; AbbVie: Research Funding; Forma: Research Funding; Takeda: Research Funding; Kite, a Gilead Company: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal